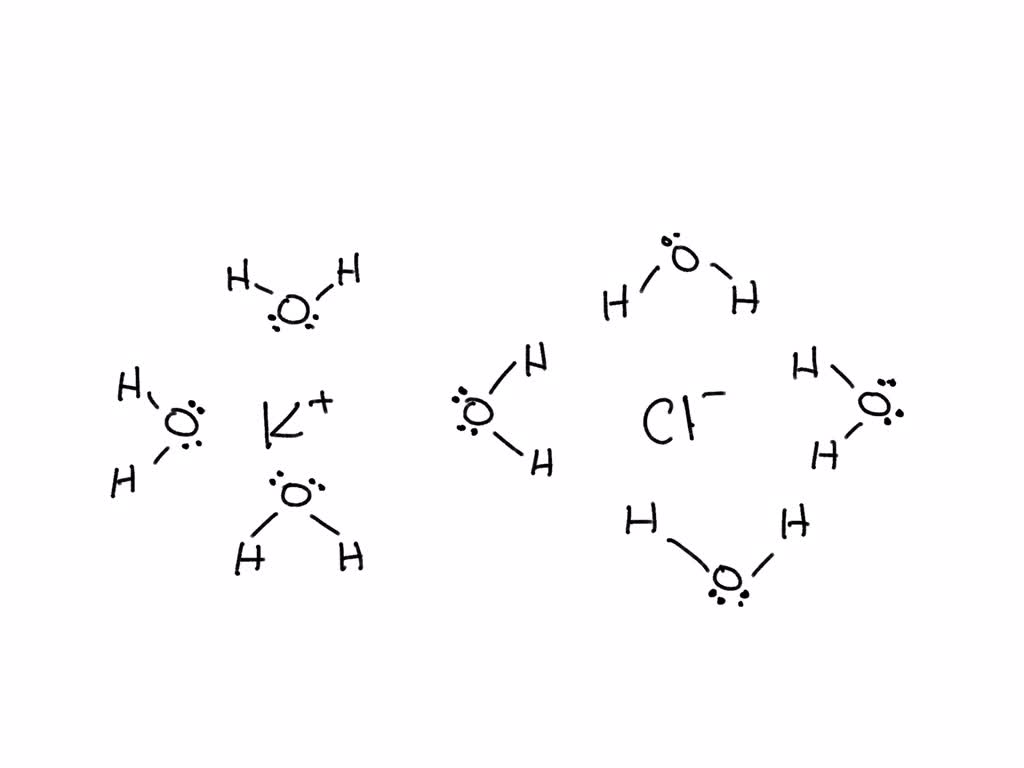
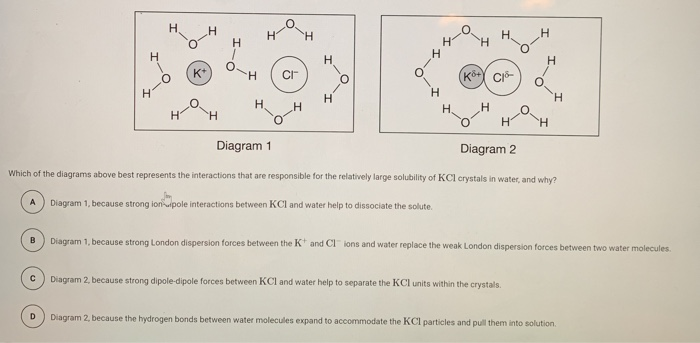
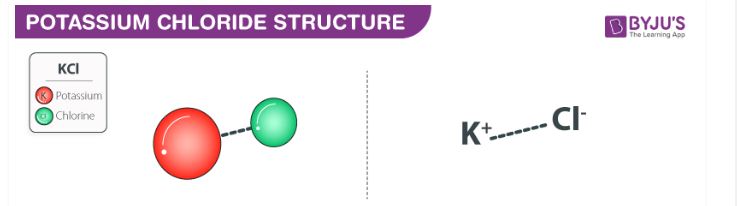
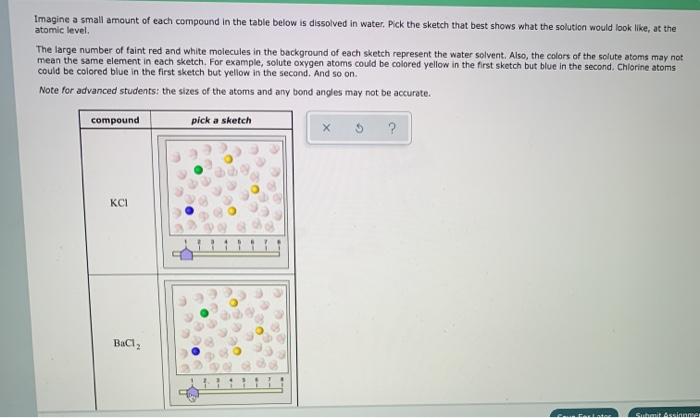
Draw the ions that form and the hydration shells that orient around them when potassium chloride (KCl) dissolves in water. Label positive and negative elementary charges and partial charges of electric dipoles.

SOLVED: Solid potassium chloride dissolves in water according to the equation below: KCl (s) ⟶ K+ (aq) + Clâˆ' (aq) ΔHº = +17.22 kJ/mol 7.50 grams of solid KCl (m.w. = 74.55

The relationship between the soluble KCl concentrations in the liquid... | Download Scientific Diagram

Chapter 13 Properties of Solutions. Consider KCl (solute) dissolving in water (solvent): –H-bonds in water have to be interrupted, –KCl dissociates into. - ppt download
A solution containing 0.5 g of KCl dissolves in 100 g of water and freezes at – 0.24° C. - Sarthaks eConnect | Largest Online Education Community
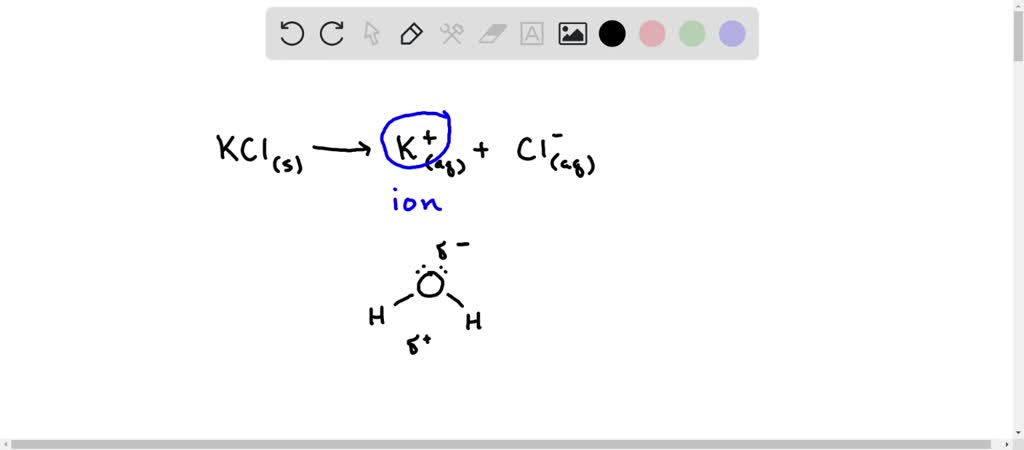
SOLVED: When KCl dissolves in water, aqueous K+ and Cl- ions result. The force of attraction that exists between K+ and H2O is called a(n) interaction. A) dipole-dipole B) ion-ion C) hydrogen

Local structures and the dissolving behavior of aqueous ammonia and its KCl and NH4Cl solutions: A Raman spectroscopy and X-ray scattering study - ScienceDirect

Solid potassium chlorate dissolves into water to form potassium and chlorate ions. What force is involved in the dissociation of \rm KClO_3 in water (intermolecular or intramolecular forces)? How would you draw